Introduction
In the ever-changing field of technology, electronic gadgets play an essential role in our daily lives by connecting people, providing convenience, and driving innovation. Behind the sleek designs of modern devices, manufacturers carefully select materials for their specific properties. This article explores the fascinating world of materials used to create electronic gadgets—from the tiniest circuits to the outer casings.
When atoms combine to form a solid crystalline material, they arrange themselves in a symmetrical pattern. In a semiconductor crystal structure, the atoms bond together through covalent bonds formed by the interaction of their valence electrons. Silicon forms such a crystalline structure and serves as a widely used semiconductor material.
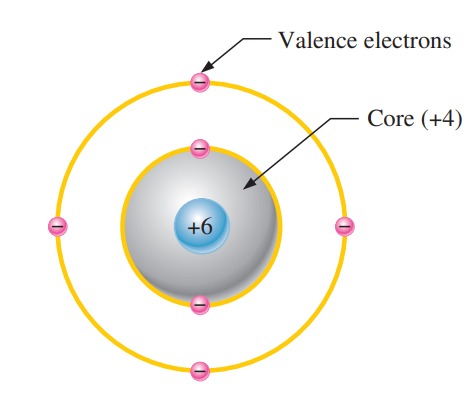
All materials consist of atoms. These atoms determine a material’s electrical properties, including its ability to conduct electrical current. When discussing electrical behavior, we represent an atom by its valence shell and a core that includes all inner shells and the nucleus. Figure 1 illustrates this concept using a carbon atom. Engineers often use carbon in certain types of electrical resistors. The carbon atom contains four electrons in its valence shell and two electrons in its inner shell. Its nucleus holds six protons and six neutrons, giving it a +6 positive charge from the protons. The core, which includes the nucleus and the two inner-shell electrons, carries a net charge of +4 (from +6 for the protons and –2 from the inner electrons).
In terms of their electrical properties, materials can be classified into three groups
- Conductors
- Semiconductors
- insulators
- Plastics and Polymers
- Printable and Flexible Materials
Now we discuss about all this three materials in detail.
1.Conductors
2.Semiconductors
3.Insulators
4.Plastics and Polymers
PE, PP, PC are lightweight and versatile polymers used in device casings, battery housings, and other structural components. PC is known for impact resistance and commonly used in laptop and smartphone bodies. PET is transparent and lightweight, often used in flexible electronics and as a display substrate.
5.Printable and Flexible Materials
Organic semiconductors enable the development of flexible and printable electronics for creating bendable displays and sensors. Graphene, composed of a single layer of carbon atoms in a hexagonal arrangement, serves as a highly conductive material known for its exceptional strength and flexibility. This material shows potential for use in future flexible electronics and high-speed electronic devices.
Conclusion
Every day, we interact with electronic devices that rely on a carefully selected range of materials for their performance, durability, and innovation. The constant search for new materials pushes the limits of what is achievable, leading to more powerful, sustainable, and versatile electronic devices in the future.