Introduction
Electrons orbit the nucleus of an atom in different energy levels. The inner electrons, located close to the nucleus, stay tightly bound to it. As the distance from the nucleus increases, the binding force weakens. The outermost electrons, called valence electrons, remain only loosely attached to the nucleus. In certain substances—especially metals like copper and aluminum—the nuclei hold these valence electrons so weakly that they break free easily. These loosely bound valence electrons become free electrons.
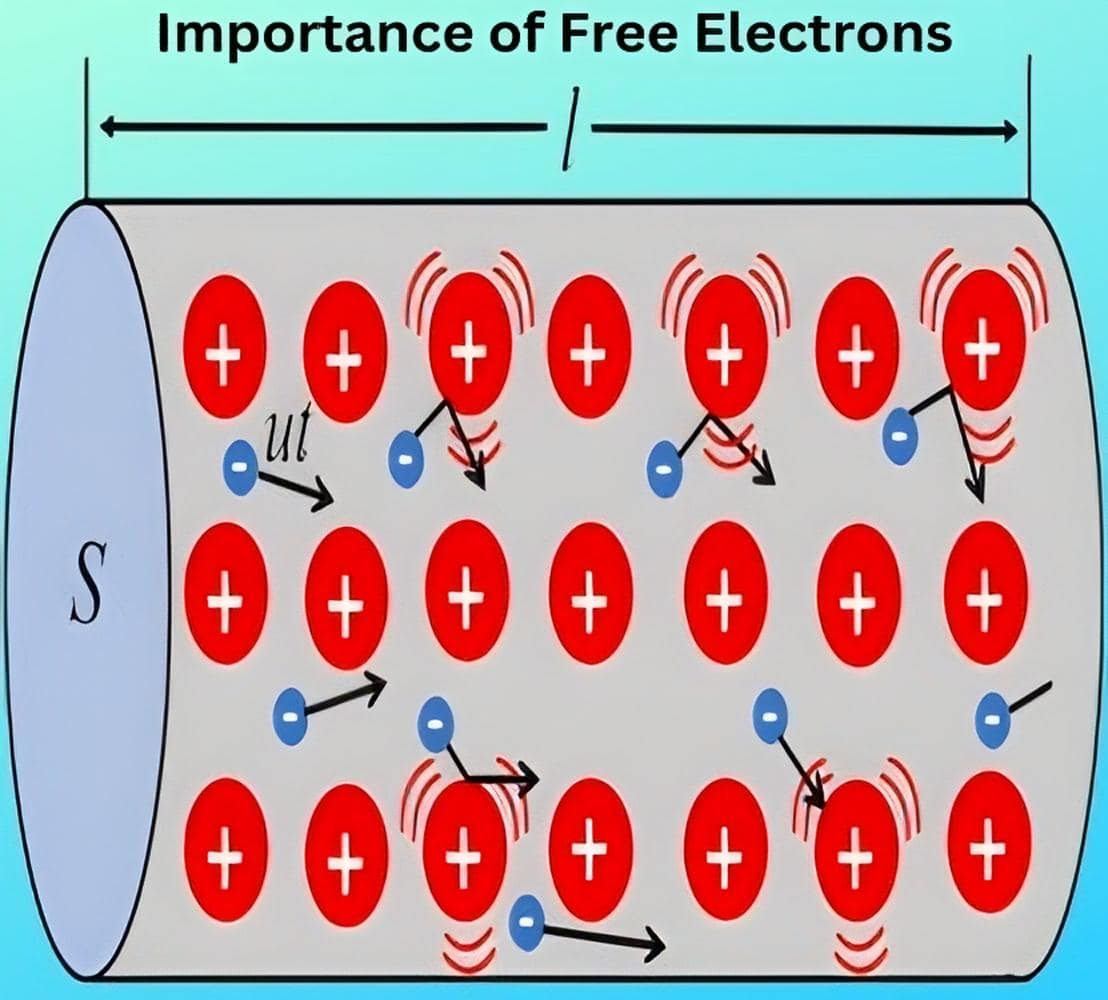
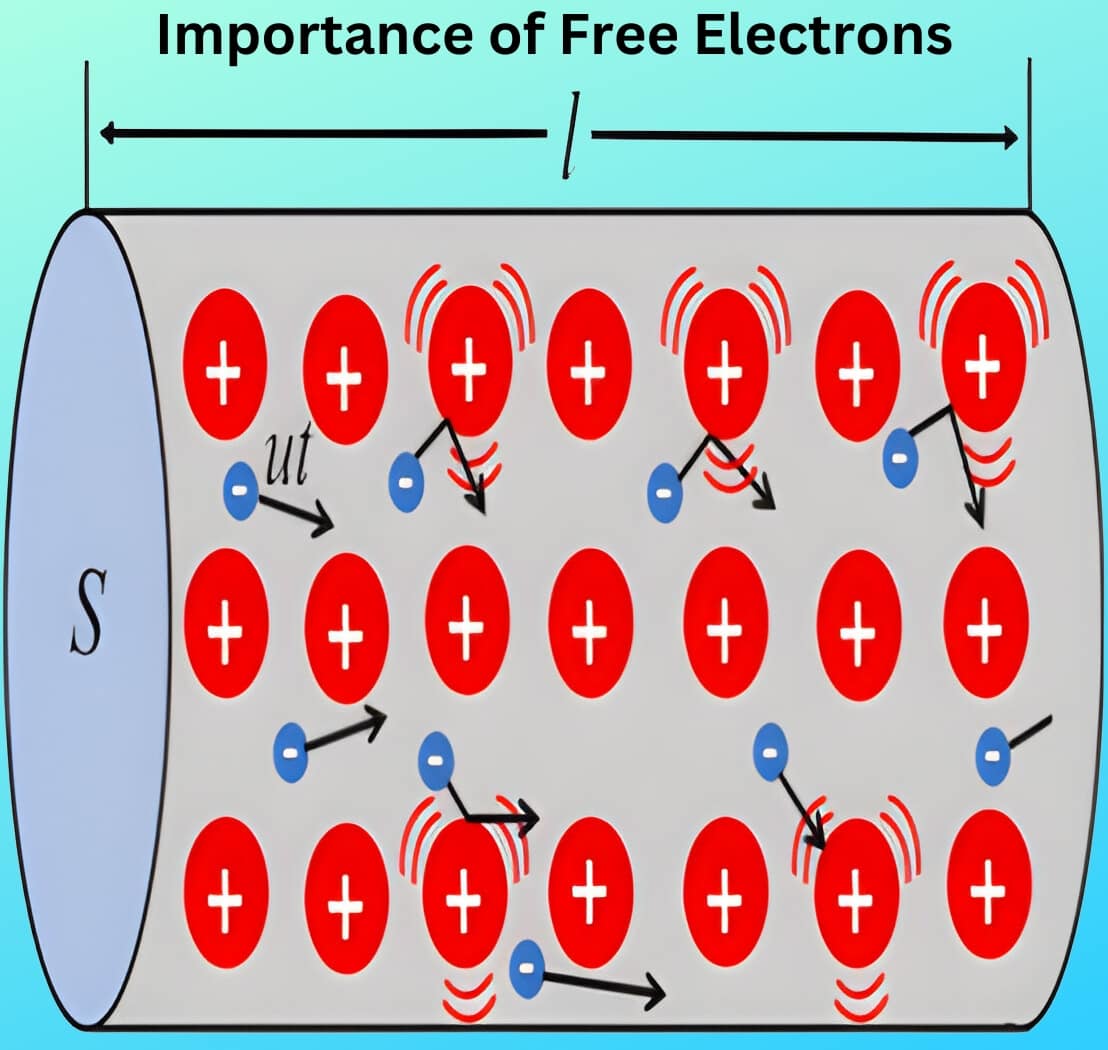
Free electrons move randomly from one atom to another within the material. In fact, they remain so loosely attached that they lose track of the atoms they originally belonged to. However, not all valence electrons in a metal behave as free electrons. Researchers have found that each metal atom can contribute at most one free electron. Since even a small piece of metal contains billions of atoms, metals contain an enormous number of free electrons. For example, one cubic centimetre of copper contains approximately 8.5 × 10^{22} free electrons at room temperature.
- A substance that contains a large number of free electrons at room temperature qualifies as a conductor of electricity—for example, all metals. When we apply a voltage source (such as a cell) across a wire made of conducting material, the free electrons readily flow through the wire and create an electric current. Silver, copper, and gold rank as the best conductors, in that order. However, since copper costs less than the others, industries commonly use it in electrical and electronic applications.
- A substance which has very few free electrons is called an insulator of electricity. If a voltage source is applied across the wire of insulator material, practically no current flows through the wire. Most substances including plastics, ceramics, rubber, paper and most liquids and gases fall in this category.Of course,there are many practical uses for insulators in the electrical and electronic industries including wire coatings, safety enclosures and power-line insulators.
- There is a third class of substances, called semi-conductors. As their name implies, they are neither conductors nor insulators. These substances have crystalline structure and contain very few free electrons at room temperature. Therefore, at room temperature, a semiconductor practically behaves as an insulator. However, if suitable controlled impurity is imparted to a semi-conductor, it is possible to provide controlled conductivity.Most common semi-conductors are silicon, germanium, carbon etc.However, silicon is the principal material and is widely used in the manufacture of electronic devices
(e.g. crystal diodes, transistors etc.) and integrated circuits.
Importance of Free Electrons
- Electrical Conductivity: As referred to earlier, loose electrons are answerable for the electrical conductivity of metals. This assets is fundamental to the operation of electrical wires, circuits, and all digital devices.
- Thermoelectricity: Free electrons also are essential in thermoelectric substances, in which they make contributions to the conversion of heat into energy. This technology is used in strength generation and waste warmth restoration.
- Semiconductors: In semiconductors, the conduct of loose electrons can be managed and modified to create digital gadgets like transistors, diodes, and incorporated circuits. These gadgets are the building blocks of modern electronics.
- Electronics and Computers: Free electrons permit the operation of digital devices together with smartphones, computer systems, and televisions. They are accountable for the drift of electric current that strategies records and performs numerous features.
- Superconductivity: In certain substances at extraordinarily low temperatures, loose electrons can shape pairs and circulate with out resistance. This phenomenon, known as superconductivity, has the potential to revolutionize energy transmission and garage technologies.
- Laser Technology: Free electrons are used inside the introduction of lasers, where their movements produce coherent mild amplification, which has a extensive range of applications in enterprise, medicinal drug, and studies.
The Electron
Electrical engineering often involves studying tiny particles called electrons. These small, negatively charged particles have negligible mass and require close examination. The following are some important properties of an electron:
- Charge on an electron, e = `1.602times10^{-19}` coulomb
- Mass of an electron, m = `9.0times10^{-31}` kg
- Radius of an electron, r =`1.9times10^{-15}` meter
The e/m ratio of an electron is 1.77 × 10^{-11} coulombs/kg. This ratio shows that an electron has very little mass compared to its charge. Because of this property, the electron moves easily and responds strongly to electric and magnetic fields.
Energy of an Electron
Valence Electrons
We call the electrons in the outermost orbit of an atom valence electrons.
The outermost orbit can have a maximum of 8 electrons i.e. the maximum number of valence electrons can be 8. The valence electrons determine the physical and chemical properties of a material. These electrons determine whether or not the material is chemically active; metal or non-metal or, a gas or solid. These electrons also determine the electrical properties of a material.
On the basis of electrical conductivity, materials are generally classified into conductors, insulators and semi-conductors.As a rough rule, one can determine the electrical behaviour of a material from the number of valence electrons as under:
- When the number of valence electrons of an atom is less than 4 (i.e. half of the maximum eight electrons), the material is usually a metal and a conductor. Examples are sodium, magnesium and aluminium which have 1, 2 and 3 valence electrons respectively.
- When the number of valence electrons of an atom is more than 4, the material is usually a non-metal and an insulator. Examples are nitrogen, sulphur and neon which have 5, 6 and 8 valence electrons respectively.
- When the number of valence electrons of an atom is 4 (i.e. exactly one-half of the maximum 8 electrons), the material has both metal and non-metal properties and is usually a semi-conductor. Examples are carbon, silicon and germanium.