Introduction
Undoubtedly, water is essential for life and, moreover, simultaneously plays a vital role in a wide range of industrial activities. Furthermore, specifically in industrial contexts, its quality and properties consistently serve as key factors in determining both efficiency and environmental impact in processes such as manufacturing and energy production. Consequently, understanding water’s role becomes crucial in optimizing industrial performance and, therefore, ensuring long-term sustainability. In this context, additionally, this blog will explore the influence of water, trace its origins, and highlight the significance of water hardness in various industrial environments.
Water is the basic necessitiy of life. It is necessary for the survival of all livings. Surprisingly and in fact, water covers approximately 80% of the Earth’s surface. Nevertheless and even so, despite this vast coverage, only 1% of the total water remains readily available. Consequently and accordingly, this limited portion must support various uses, including domestic, agricultural, municipal, and industrial work. Therefore and hence, efficient management becomes essential.
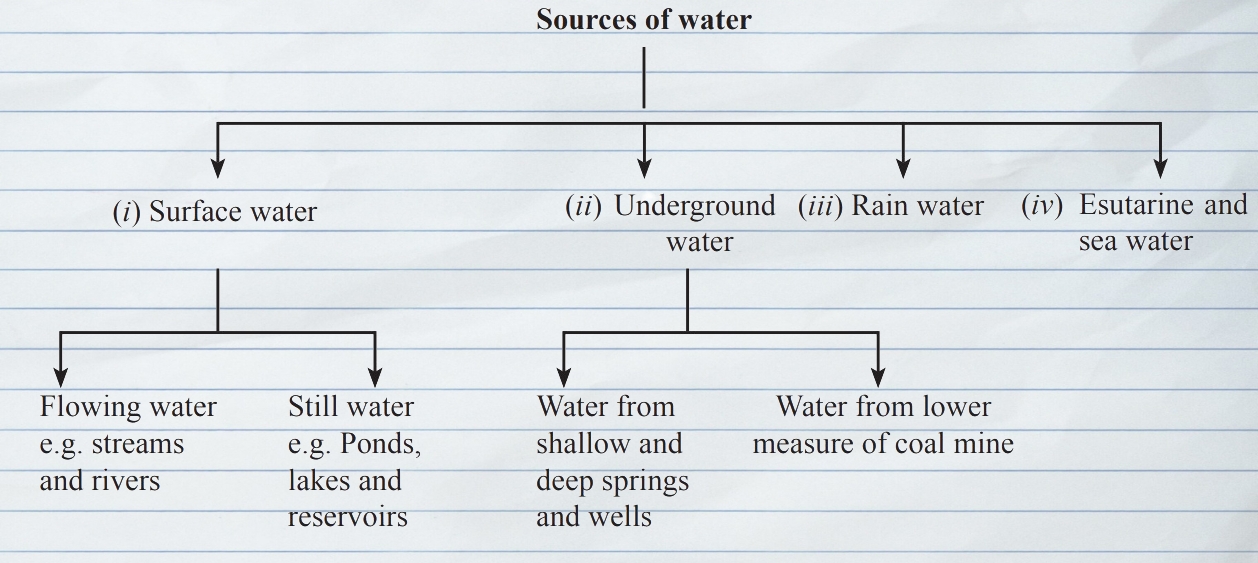
Source of water
Types of impurities found in water
(1) Dissolved impurities
(2) Suspended impurities
(3) Dissolved impurities
(4) Bacterial impurities
| Purpose | Specifications and Remark |
|---|---|
| Paper Industry | (a) Free from alkalinity (alkaline water consumes more alum, thereby increasing the cost of production). |
| (b) Free from hardness: (Calcium and magnesium salts increase the ash content of the paper produced). |
|
| (c) Free from colour, turbidity and salts of Fe and Mn : (colour and brightness of the paper are affected by the above impurities). |
|
| (d) Free from Silica : (Silica causes cracks in the paper). | |
| Textile industry | (a) Free from turbidity : (turbidity causes uneven dyeing). |
| (b) Free from colour, and salts of Fe and Mn : (these impurities cause stains on the fabric). |
|
| (c) Free from hardness and organic matter : (Hard water reduces the solubility of acidic dyes and causes precipitation of basic dyes. They also render the dyeing non-uniform. Organic matter may cause foul smell of the product). |
|
| Thermal Power Generation industry | (a) Boiler feed Water : Free from hardness : (hard water causes scaleformation on boiler metal surface, thereby reducing heat transfer efficiency and causing shut-down or even accidents). |
| (b) Cooling water : The water should be non-scale forming, non-corrosive, and should not permit the growth of algae. Scale and algae reduce the heat transfer efficiency and interfere with free flow of water. |
|
| Dairy industry | The water should be colourless, odourless, and tasteless. It should be free from pathogenic organisms. |
| Beverage industry | The water should be pure. It should not be alkaline, because alkalinity in water tends to neutralise the fruit acids and distorts the taste. |
| Laundry | The water should be free from colour, hardness and salts of Fe and Mn : (Hardness of water increases the consumption of soaps and detergents. Fe and Mn salts impart undesirable colour to the fabric. |
| Ice making, brewing, canning and distillery industry | Free from hardness and bacteria. |
Hardness of water
The waters which do not produce lather or produces very little lather with soap are known as hard water. On the other hand soft waters readily produces a lot of lather when mixed with a little of soap. Therefore the study of hardness of water has great importance.
✷Hardness:Originally, hardness was defined as the soap-consuming capacity of a water sample. This is because soaps generally consist of the sodium salts of long-chain fatty acids, such as oleic acid, palmitic acid, and stearic acid. In most cases, the soap-consuming capacity of water is mainly due to the presence of calcium and magnesium ions. Specifically, these ions react with the sodium salts of long-chain fatty acids present in the soap to form insoluble scums of calcium and magnesium soaps, which, in turn, do not possess any detergent value. Therefore, water containing these ions is considered hard, as it reduces the effectiveness of soap.
`2C_{17}H_{35}COONa+CaCl_2rightarrowleft(C_{17}H_{35}COOright)_2Ca+2NaCl`
Soap (soluble)Calcium soap (insoluble) Other metal ions like `Fe^{+2}`, `Mn^{+2}` and `Al^{+3}` also react with the soap in the same fashion, thus contributing to hardness but generally these are present in natural waters only in traces. Further, acids such as carbonic acid can also cause free fatty acid to separate from soap solution and thus contribute to hardness. However, in practice, the hardness of a water sample is usually taken as a measure of its `Ca^{+2}` and `Mg^{+2}` content.
Degree of Hardness
Types of Hardness
(i) Temporary Hardness:
To begin with, temporary hardness arises from the presence of calcium and magnesium bicarbonates. As a matter of fact, people commonly refer to it as carbonate hardness or alkaline hardness. In addition, this type of hardness disappears when one boils the water. When heated, calcium and magnesium bicarbonates break down into insoluble carbonates or hydroxides. As a result, these compounds form precipitates. After that, one can remove the solids through filtration. Therefore, treating temporary hardness is relatively simple compared to permanent hardness. In conclusion, understanding this process helps in selecting the correct water softening method.Ultimately, understanding this process helps in choosing the appropriate water softening method.
`\left(C_aHC_o3\right)\rightarrow\left(\triangle C_aCO_3+H_2O+CO_2M_gHCO_{32}\right)\rightarrow\left(\triangle M_gOH_2\right)`
(ii) Permanent Hardness:
Specifically, this type of hardness occurs due to the presence of chlorides and sulphates of calcium, magnesium, iron, and other heavy metals such as Al₂(SO₄)₃. Consequently, it is also known as non-carbonate or non-alkaline hardness. In contrast, one cannot remove it by simply boiling the water, unlike temporary hardness. However, various chemical agents can effectively eliminate it.
Units of Hardness
1. Parts per million (ppm)
2. Milligrams per litre (mg/L)
3. Degree French (°Fr)
4. Degree Clark (°Cl)
Relationship among various units of hardness
Conclusion
Clearly, water is a crucial element in industrial processes, as it directly connects operations, efficiency, and environmental impact. In this regard, knowledge of water sources, quality, and hardness is essential for industries to prosper sustainably. Therefore, responsible water management practices are necessary to ensure a balance between industrial advancement and environmental conservation.