Introduction
Water is an important resource that is crucial for a variety of industrial and household uses. Hard water, which contains high levels of minerals, can lead to problems like scale buildup and decreased efficiency of appliances. Cold lime-soda softeners are a dependable option for dealing with hard water issues.This blog will explore the various kinds of cold lime-soda softeners and their distinct features.
Types of cold lime-soda softeners for Your Water Treatment Needs
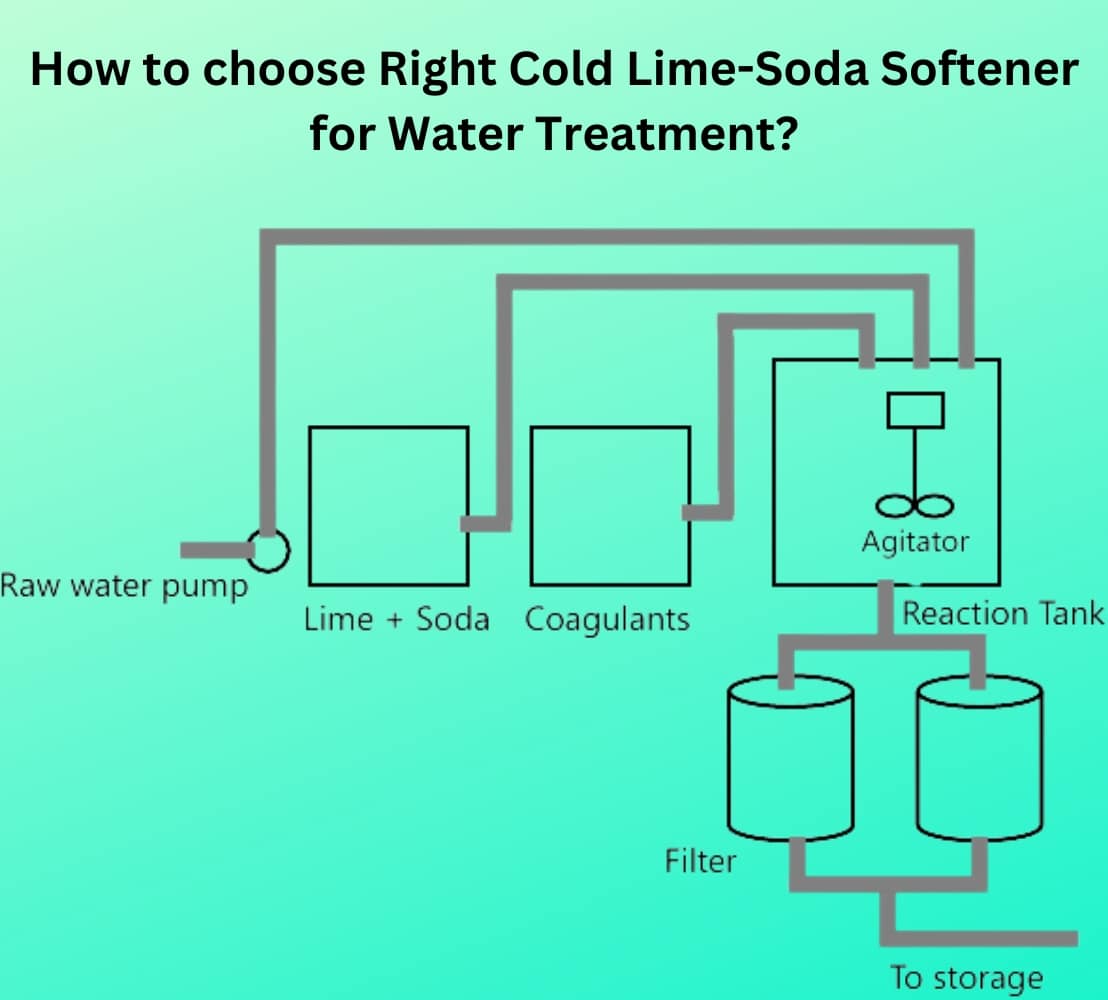
(1) Intermittent or batch process
- An intermittent-type cold lime-soda softener uses two tanks operating alternately to soften water. Each tank has inlets for raw water and chemicals, outlets for softened water and sludge, and a mechanical stirrer, as shown in Fig. 1.
-
Operators slowly introduce raw water and calculated amounts of chemicals into the tank while the stirrer continuously agitates the mixture. To speed up the reaction, they also add a portion of sludge from a previous operation. This sludge serves as a nucleus for fresh precipitation and accelerates the softening process.
- By the time the tank fills, the chemicals have nearly completed their reactions. Operators stop the stirring and allow the sludge to settle at the bottom. They collect the clear, softened water through a float pipe and direct it to the filter. They also remove the settled sludge through the sludge outlet. By operating two tanks in alternating cycles, they maintain a continuous supply of softened water.
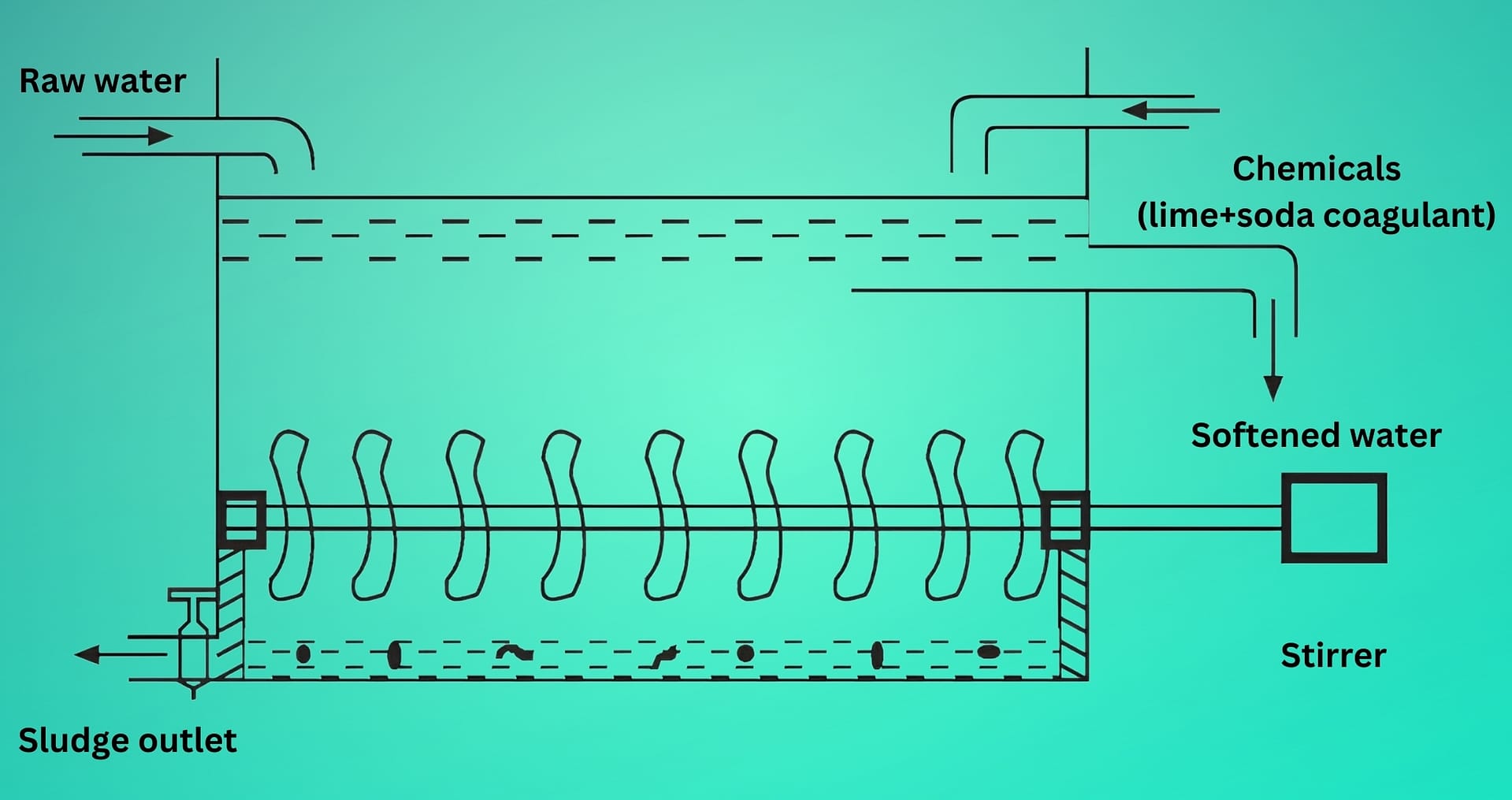
(2) Conventional type
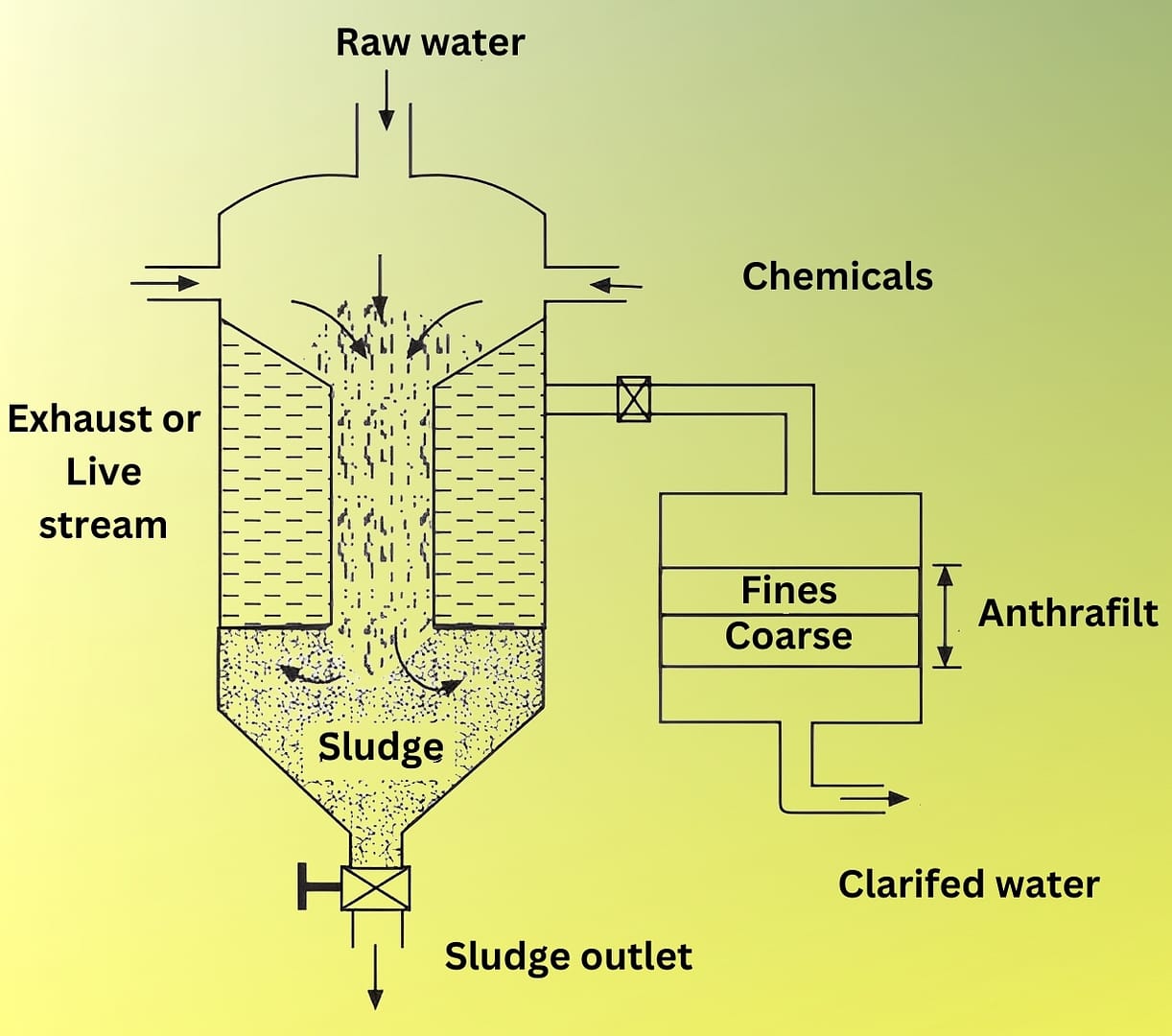
- In this process, the raw water and the chemicals in calculated quantities are continuously fed from the top into an inner chamber of vertical circular tank provided with a paddle stirrer (Fig. 2).
- As the raw water and chemicals flow down the chamber, they come into close contact and undergo softening reactions. The process forms sludge, which settles at the bottom of the outer chamber. Operators periodically remove this sludge through the sludge outlet. Meanwhile, the softened water rises and flows through the fiber filter, where it loses any remaining sludge particles. The filtered, softened water then exits through the outlet.
- Water treated by the cold lime-soda process generally produces softened water containing about 50 – 60 ppm of residual hardness.
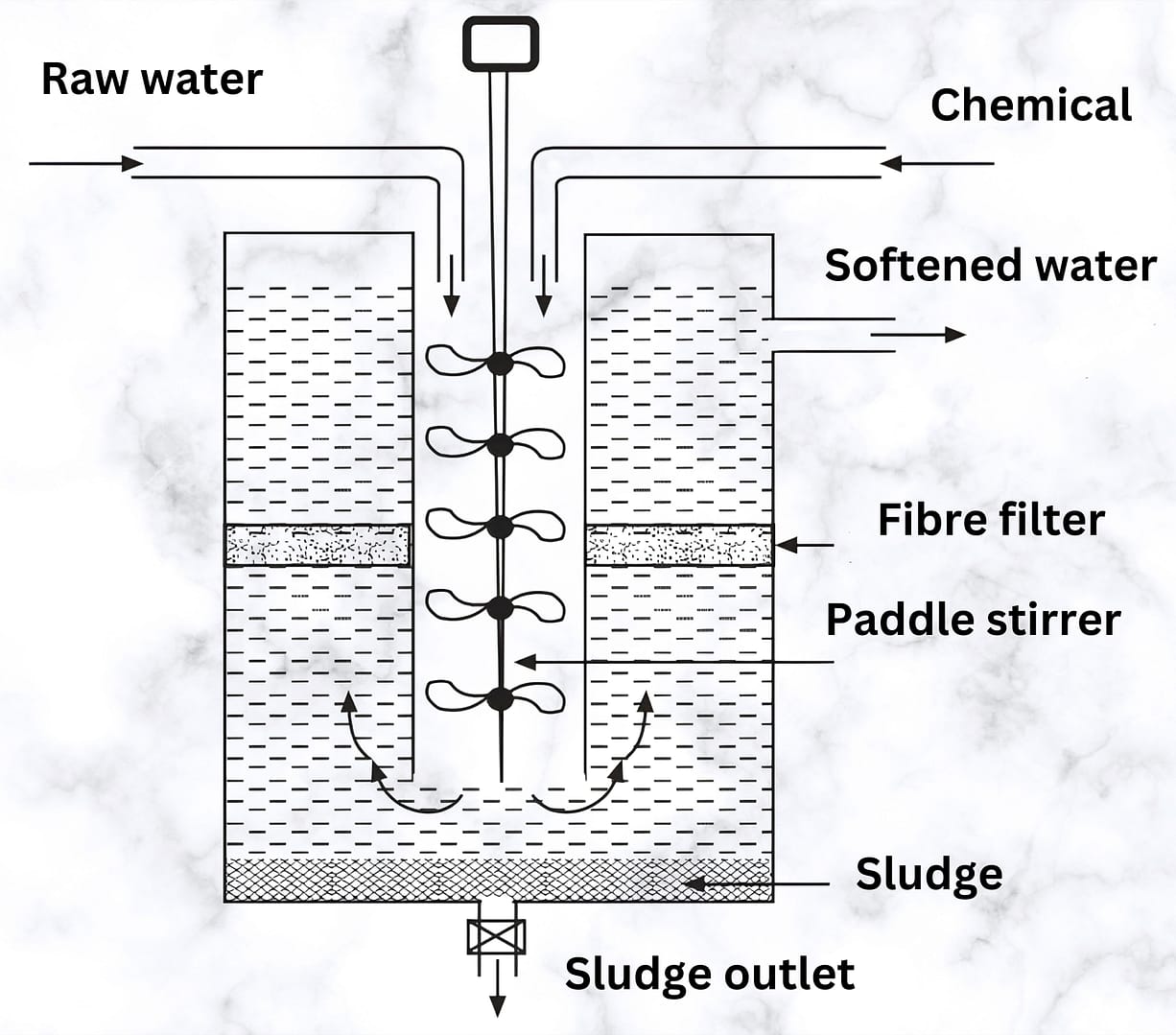
(3) Catalyst or spiractor type
- The spiractor contains a conical tank, and operators fill about two-thirds of it with finely divided granular catalyst (Fig. 3). They may use either an open tank for gravity operation or a closed tank for operation under pressure.
- In both cases, operators allow the raw water and calculated quantities of chemicals to enter the tank tangentially near the bottom of the cone. The mixture then spirals upward through the suspended catalyst bed. They use a finely granulated (0.3 to 0.6 mm diameter), insoluble mineral substance as the catalyst—such as graded calcite, sand, or green sand.
- The process takes about 8 to 12 minutes of retention time. During the softening reactions, sludge forms and adheres to the catalyst grains, causing the granules to grow in size. The softened water rises to the top, and operators draw it off from there.
-
Engineers favor the catalyst, or spiractor-type, continuous water softener because it produces granular sludge that drains and dries quickly, making it easy to handle.
(4) The sludge blanket type
- Operators widely use sludge blanket-type water treatment equipment for coagulation, settling, and water softening using the cold lime-soda process. These softeners differ from conventional types because they filter the treated water upward through a suspended sludge blanket made of previously formed precipitates. As a result, a single unit carries out all three processes—mixing, softening, and clarification.
- In conventional equipment, some of the added lime suspension settles with the sludge before it has time to dissolve and react with the hardness-causing impurities in the raw water. This process wastes a portion of the lime. In contrast, the sludge blanket type prevents this waste. Its upward filtration through the suspended sludge blanket ensures complete utilization of the added lime.
- In conventional equipment, operators often observe the formation of precipitates or deposits on granules, filter media, pipelines, or distribution systems that carry the filtered effluent. To prevent these deposits, they typically perform recarbonation using CO₂.
- In the sludge blanket type of equipment, the treated water remains in close contact with a large mass of solid phase, which mostly prevents supersaturation and the formation of after-deposits. This process produces an effluent clear enough—usually with turbidity less than 10 mg/L—for many industrial applications. As a result, operators often skip subsequent filtration.
- The sludge blanket type of equipment requires only a one-hour retention period, compared to four hours in conventional units. It also removes silica more effectively. Because of its higher efficiency, shorter detention time, and smaller space requirements, the sludge blanket system is rapidly replacing conventional water softening equipment.
Hot lime-soda process for Your Water Treatment Needs
- The reactions involved in water softening occur in very dilute solutions—around 0.001 M—and therefore proceed slowly. To accelerate these precipitation reactions, operators raise the temperature. Doing so increases both the rate of the ionic reactions and the speed at which particles of measurable size form.
- Figure 1.4 shows how temperature affects the speed and completeness of precipitation reactions involved in removing scale-forming constituents. At 96°C, the system completes precipitation within 10 minutes—faster than it does after several hours at just 10°C. This effect becomes especially noticeable when precipitating magnesium compounds.
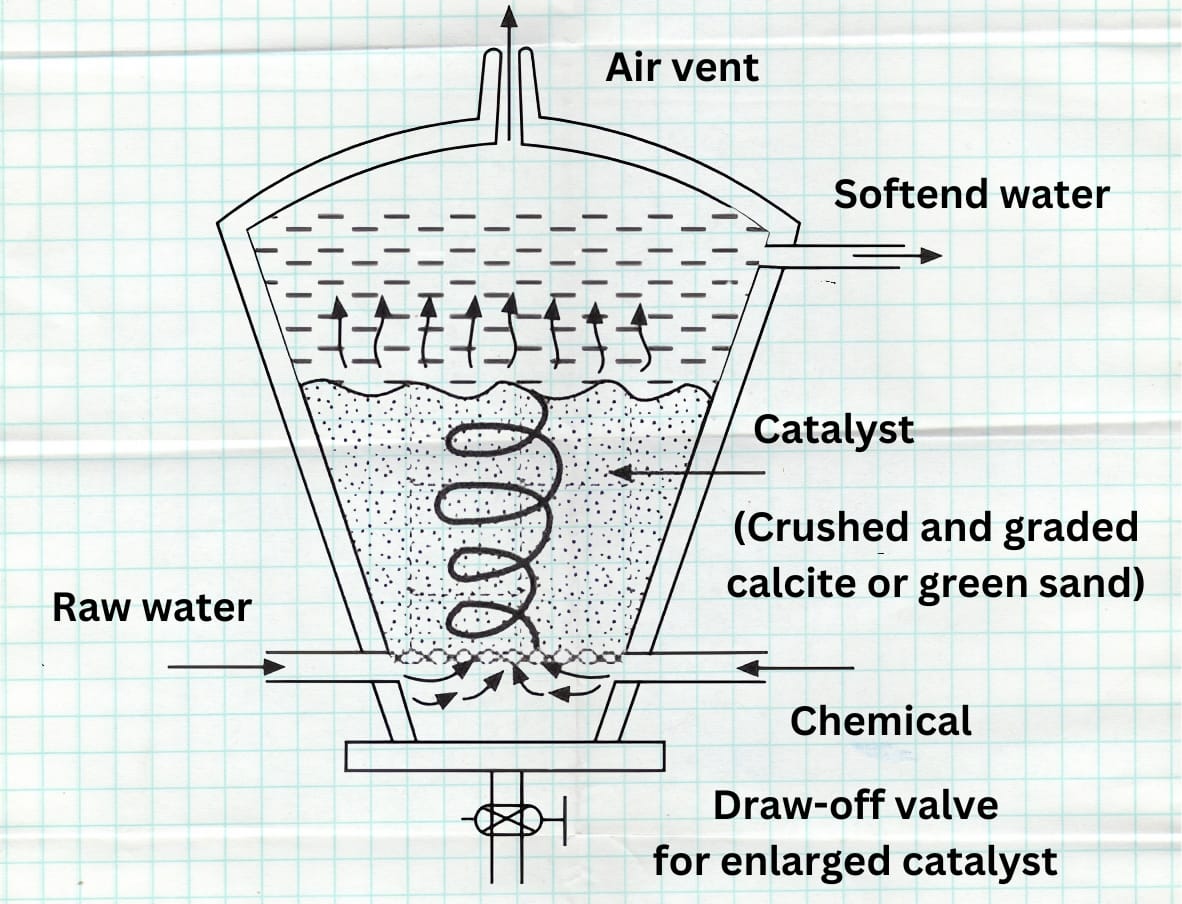
- Hot lime-soda plants carry out softening at 94° – 100°C which has several advantages.For efficient softening, cold lime-soda softening plants must be of considerable area and water-storage capacity, whereas hot lime-soda softeners are much more rapid in operation and therefore for a given through-put, much more compact. Elevated temperatures not only accelerate the actual chemical reactions but also reduce the viscosity of the water and increase the rate of aggregation of the particles.
- As a result, both settling and filtration rates increase. The hot lime-soda process achieves a much higher softening capacity compared to the cold process. Because the sludge settles quickly, operators don't need to add coagulants. They also use a smaller excess of chemicals than required in the cold process. Additionally, the high temperature drives out some of the dissolved gases. The hot lime-soda process produces softened water with relatively lower residual hardness—around 17 to 34 ppm—compared to 50 to 60 ppm in the cold process.
- A typical hot lime soda water softening unit is shown in Fig. 4, which includes a reaction cum settling tank and a filte . If the water is alkaline, filtration through sand and gravel beds might contaminate the water with dissolved silica, particularly if the quartz used is of inferior quality.
- Other filtering media used are anthracite coal, calcite and magnetite.If the precipitation is incomplete in the softening tank,“after-precipitation” occurs in pipes, storage tanks and even in boiler itself. If slight excess of chemicals are used over that theoretically required, more rapid and more complete removal of hardness will result.But if larger excess of chemicals are used, naturally they will appear in the softened water. Lime soda plants do not produce water of zero hardness.
- Tips for the solvening problems on water treatment by Lime-soda process.On the basis of the various reactions taking place in lime-soda process given earlier, the following deductions can be made:
(i) One equivalent of calcium temporary hardness requires one equivalent of lime
(ii) One equivalent of magnesium temporary hardness requires two equivalents of lime
(iii) One equivalent of calcium permanent hardness requires one equivalent of soda,
(iv) One equivalent of magnesium permanent hardness requires one equivalent of lime and one equivalent of soda.
(v) Lime reacts with HCl, `H_2so_4`, `Co_2`, `H_2s`, salts of iron,aluminium, etc. Accordingly, their respective equivalents must be considered for calculating the lime requirement.
(vi) Lime, while reacting with HCl, `H_2so_4`, `Mgso_4`,`Mgcl_2`,
`Mgleft(no_3right)_2`, salts of Fe, Al etc., generates the corresponding quantities of calcium permanent hardness. Accordingly, these constituents also should be considered while calculating the soda requirement.
(vii) Two equivalents of `Hco_3` reacts with two equivalents of lime as follows:
calculations for soda requirement.
For solving numerical problems on lime-soda requirements for softening of hard water, the following steps may be followed:
- The units in which the impurities analysed are expressed i.e., ppm (or mg/l), grains per gallon (or degrees Clark), etc., are to be noted.
- Substances which do not contribute towards hardness (e.g., KCl, NaCl,`Sio_2`,`Na_2So_4`, `Fe_2o_3`,`K_2So_4`, etc.) should be ignored while calculating lime and soda requirements. This fact should be explicitly stated.
- All the substances causing hardness should be converted into their respective `Caco_4` equivalent, as a matter of convention and convenience.
`Ca\left(CO_3\right)` =(Weight of the impurity Chemical equivalent weight of the impurity)×50
(since chemical equivalent weight of `Ca\left(CO_3\right)` = 50).
For instance, 136 parts by weight of `Ca\left(SO_4\right)` would contain the same amount of Ca as that of 100 parts by weight of `Ca\left(CO_3\right)`. Hence, in order to convert the weight of `Ca\left(SO_4\right)` as its `Ca\left(CO_3\right)` equivalent,
the weight of `Ca\left(SO_4\right)` should be multiplied by a factor of `frac100 136` or `frac50 68`.
Conclusion
Choosing the correct Cold Lime-Soda Softener is essential for successful water softening and preserving the functionality of water-using devices. Taking into account factors like water hardness, flow rate, space needed, operational expenses, automation capabilities, environmental effects, and vendor credibility allows for a well-informed decision that suits individual water treatment requirements. Purchase a top-notch Cold Lime-Soda Softener to guarantee a consistent flow of softened water, supporting the durability of plumbing and appliances while aiding in sustainability efforts.