Introduction
Coal, a dark sedimentary rock created from ancient plant remains, has been pivotal in shaping the world's energy scene. It has served as a vital power source for generations, driving the industrial revolution and aiding in global economic growth. Yet, the utilization of coal poses various environmental, social, and health issues, prompting continuous discussions about its place in the constantly changing energy landscape.
Understanding Elemental Composition of a coal
1. Carbon (C):
2. Hydrogen (H):
3. Nitrogen (N):
4. Sulfur (S):
5. Oxygen (O):
characteristics, and the overall chemical balance in various engineering applications.
Applications of Elemental Composition in Chemical Process Optimization
1. Fuel Characterization
Ultimate Analysis plays a key role in evaluating a fuel’s elemental content. By carefully examining the carbon, hydrogen, and sulfur content, chemical engineers gain a detailed understanding that helps them optimize combustion, improve energy efficiency, and develop cleaner energy resources.
2. Material Synthesis and Design
Initially, in material science, Ultimate Analysis guides engineers; indeed, they control elemental percentages, furthermore managing polymers. Consequently, this knowledge, therefore, is integral; additionally, it aids in developing properties. Moreover, engineers apply it, thus ensuring functionality. Meanwhile, research expands, similarly advancing techniques. Hence, Ultimate Analysis is valuable, besides practical, ultimately supporting progress.
3. Environmental Compliance
On the other hand, environmental management and keeping it at the minimal level become crucial valuess in chemical engineering. Ultimate Analysis measures help to control the proportions of sulfur and nitrogen in emissions; achieving this goal, the company avoids being penalized for violations of the existing regulatory framework and in general contributes to the development of green manufacturing.
4. Process Efficiency and Quality Control
Elemental composition of raw material and the products is of immeasurable importance in optimizing the chemical reaction. In chemical engineering, Ultimate Analysis helps engineers create optimized processes that minimize waste and ensure strong quality control.
Classification and rank of coal
| Fuel | Moisture of air dried sample at 40 °C (%) | C (%) | H (%) | N (%) | O (%) | Calorific value (kcal/kg) |
|---|---|---|---|---|---|---|
| Wood | 25 | 50 | 6 | 0.5 | 43.5 | 4000-4500 |
| Peat | 25 | 57 | 5.7 | 2 | 35.3 | 4125-5400 |
| Lignite | 20 | 67 | 5 | 1.5 | 26.5 | 6500-7100 |
| Sub-bituminous coal | 11 | 77 | 5 | 1.8 | 16.2 | 7000-7500 |
| Bituminous coal | 4 | 83 | 5 | 2 | 10.0 | 8000-8500 |
| Semi-bituminous coal | 1 | 90 | 4.5 | 1.5 | 4.0 | 8350–8500 |
| Anthracite | 1.5 | 93.3 | 3 | 0.7 | 3.0 | 8650–8700 |
Analysis of Coal
- Proximate analysis
- Ultimate analysis
1.Proximate Analysis
1.Moisture Content
A known weight of coal (air-dried) is taken in a crucible and heated in an electric hot air oven at about 105 °C–110 °C for about one hour. After 1 hour, it is taken out from the oven and cooled in a dessicator and weighed. Loss in weight of the sample is found out and the percentage of moisture is calculated as follows:
=Loss in weight of the sample or weight of the moistureWeight of the coal sample taken × 100
Significance
- High moisture content is undesirable because it reduces the calorific value and increases the transportation cost.
- Presence of excessive moisture quenches fire in the furnace.
- A considerable amount of heat is wasted in evaporating the moisture during combustion.
2.Volatile Matte
The volatile matter present in the coal may be combustible gases such as `H_2`,CO,`CH_4` and other hydrocarbons or non-combustible gases such as `CO_2` and `N_2` . It does not include moisture of the coal.
It is determined by heating a known weight of moisture-free coal in a silica crucible covered with a vented lid at 950 ± 20 °C for 7 minutes in a muffle furnace. The crucible is then taken out and cooled inside a dessicator and weighed again. Complete removal of volatile matter is judged by bubbling the gas through a water seal. Loss in weight gives the weight of the volatile matter and the percentage of volatile matter is calculated as follows:
Weight of volatile matter = (`W_1` - `W_2`) g%
Volatile matter = `frac{W_1-W_2}W` × 100=Loss in weight due to removal of volatile matter × Weight of the coal sample taken × 100
It may be noted here that it is not correct to say coal with 20% volatile matter or coal containing 20% volatile matter because volatile matter is not present as such in coal but it is the product of thermal decomposition of coal; hence, it should be accurately described as coal yielding 20% volatile matter although the former term is widely used in coal analysis.
Significance
- Coal containing high percentage of volatile matter burns with a long flame and high smoke and has low calorific value. Coals with high percentage of volatile matter ignite easily but burn very quickly.
- A high percentage of volatile matter indicates that a large proportion of fuel is burnt as gas.
- Presence of non-combustible gases is undesirable since they do not add to the heat value.
- For efficient use of fuel, the outgoing combustible gas (volatile matter) has to be burnt by supplying secondary air. This requires a large combustion space.
- If the furnace volume is small or flame is short, a large proportion of volatile matter will escape unburnt.
- Coals with higher percentage of volatile matter do not cake well, whereas medium volatile coals containing 20–30% of volatile matter are capable of forming hard and strong coke on carbonisation.
- Low volatile matter containing coals do not cake at all and are thus unsuitable for coke making.
- High volatile matter is desirable in coal gas manufacture because volatile matter in a coal denotes the proportion of coal that will be converted into gas and tar products by heat.
- However, for the manufacture of metallurgical coke, coal with low volatile matter content is preferred.
3.Ash
Ash is the non-combustible, useless matter that is left behind when all the combustible substances have burnt off from coal. Ash usually consists of silica, alumina, iron oxide and small quantities of lime, magnesia, etc.
You determine the ash content by heating the residue (after removing moisture and volatile matter) at 700 ± 50 °C for half an hour without a lid in a muffle furnace. After heating, you cool the residue in a desiccator and weigh it. You then calculate the percentage of ash using the weight of the residue as follows.
% ash = `frac{W_3}W` × 100 =Weight of ash formed × Weight of the coal sample taken × 100
Significance
- High percentage of ash is undesirable as it reduces the calorific value of the fuel.
- Presence of ash increases the transporting, handling and storage cost.
- It also involves additional cost of ash disposal.
- Fused ash lumps (clinkers) block the interspaces of the grate on which coal is being burnt. This causes obstruction in air supply. Hence, burning of coal becomes irregular. Hence, lower the ash content better the quality of the coal.
4.Fixed Carbon
Fixed carbon is the material remaining after determination of moisture, volatile matter and ash content. It is determined indirectly by the formula:
Percentage of fixed carbon = 100 – percentage of (moisture + volatile matter + ash).
Significance
2.Ultimate analysis
1.Carbon and Hydrogen
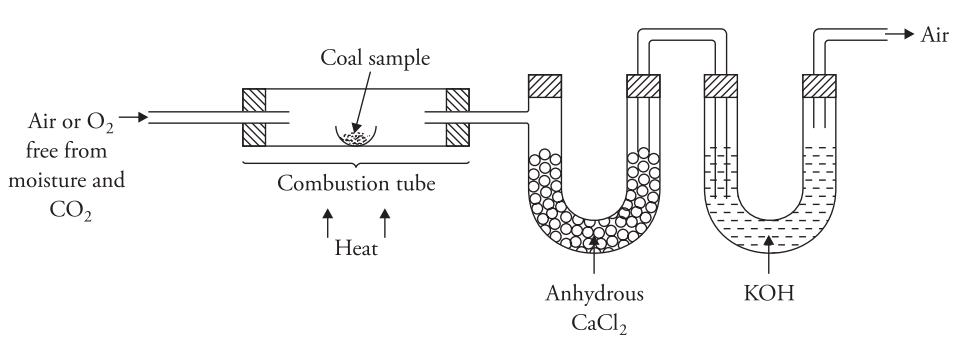
Carbon in coal sample occurs in combined form in complex organic matter and can also be present as mineral carbonates.Hydrogen on the other hand is found in organic matter in coal and is also found associated with the moisture present in coal. To estimate carbon and hydrogen accurately weighed coal sample is burnt in a combustion tube in a current of `O_2` (free from `CO_2` and moisture).
The carbon and hydrogen present in the fuel is converted to `CO_2` and `H_2O`,respectively.These are then absorbed by previously weighed tubes containing KOH and anhydrous `C_aCl_2` The increase in weights of these tubes gives the amount of `CO_2` and `H_2O`formed.The percentage of C and H is then calculated as follows.
= `frac{2}{18}` × Increase in weight of CaCl2 tubeWeight of coal sample taken× 100
Significance
- Calorific value of a fuel is directly related to the carbon content; hence, greater the percentage of carbon, greater is the calorific value of the fuel.
- Percentage of carbon increases from lignite to anthracite;thus, the percentage of carbon forms the basis of classification of coal.
- High percentage of hydrogen also increases the calorific value of coal. However, hydrogen is mostly associated with volatile matter and affects the use to which coal is put.
- In carbonisation and gasification industries, hydrogen of coal is responsible for the production of many useful materials such as gaseous hydrogen, methane, etc.
2.Nitrogen
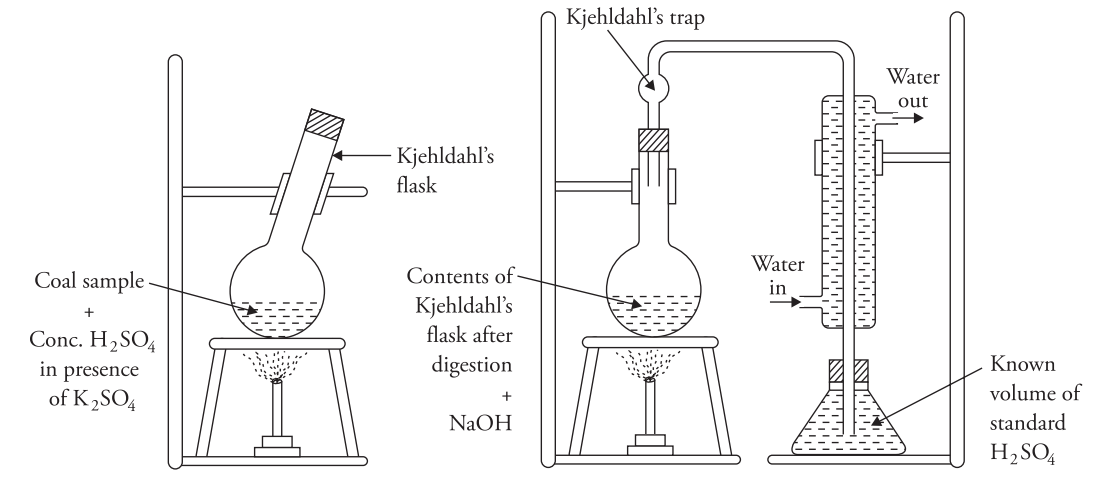
- You accurately weigh the coal sample and heat it with concentrated
H₂SO₄in the presence ofK₂SO₄using a long-necked Kjeldahl’s flask. The nitrogen present in the coal converts to(NH₄)₂SO₄during this process. - Once you obtain a clear solution (indicating complete conversion of nitrogen into ammonium sulphate), transfer the contents into a round-bottom flask. Then, heat the solution with excess
NaOHto liberateNH₃. - The liberated
NH₃is absorbed in a known volume of standardN/10H₂SO₄solution. - You determine the volume of unused
H₂SO₄by titrating it against a standardN/10NaOH solution. The difference gives you the volume of acid used. Using the volume of acid neutralized byNH₃, you can calculate the percentage of nitrogen in the sample.
`NH_3` formed
`NH_3` dissolved in 1 liter water)
g `NH_3` = 14 g nitrogen.
= `frac{14}{1000}` × `N_1V_1` nitrogen
Significance
- Since nitrogen is an inert and incombustible gas with no calorific value, its presence in fuel is undesirable.
3.Sulphur
Sulphur is found in coal in three forms, as organic sulphur compounds, as inorganic sulphides and also as inorganic sulphates.To estimate the amount of sulphur in the coal sample a known amount of coal is burnt completely in a bomb calorimeter in a current of oxygen. Sulphur in the coal is oxidised to sulphates.
You extract the ash from the bomb calorimeter using dilute hydrochloric acid. Then, you treat the acid extract with BaCl₂ solution to precipitate the sulphate as BaSO₄. Next, you filter, wash, dry, and heat the BaSO₄ precipitate to a constant weight. You can then estimate the sulphur content using the weight of the BaSO₄ formed as follows:
S→`O_2\left(SO_4\right)_2`→`Ba\left(Cl_2\right)Ba\left(SO_4\right)`
Weight of
`Ba\left(SO_4\right)`=xg
∵ 233g of `Ba\left(SO_4\right)`=32g of S
∴ xg of `Ba\left(SO_4\right)`=32233 × [x]
%S=32233 × xw × 100 or %S=32233×Weight of `Ba\left(SO_4\right)`× Weight of coal taken×100
Significance
- Although sulphur increases the calorific value of fuel, its presence is undesirable because it oxidizes to
SO₂andSO₃, which cause environmental pollution. - Sulphur-containing coal is not suitable for preparing metallurgical coke. Presence of sulphur in coke used in the iron industry affects the quality and properties of steel.
4.Ash
- You determine it in the same way as in proximate analysis.
5.Oxygen
Oxygen occurs in both the organic and inorganic portions of coal. You determine its percentage indirectly by subtracting the percentages of carbon, hydrogen, nitrogen, sulfur, and ash from 100%, using the formula:
% of oxygen = 100 − % (C + H + N + S + ash).
Significance
- Oxygen is present in coal in combined form. It is present in association with hydrogen; hence, it reduces the hydrogen available for combustion.
- Moreover, high oxygen-containing coals have high inherent moisture and hence low calorific values.
- Calorific value decreases about 1.7% for every 1% increase in oxygen.Thus, a good-quality coal should have low percentage of oxygen.
Conclusion
Ultimate Analysis stands as a cornerstone in the field of chemical engineering, providing engineers with a lens to scrutinize and optimize the elemental composition of substances. By exploring the chemical landscape carefully, chemical engineers drive innovation, improve efficiency, and contribute to a sustainable future. Ultimately, Ultimate Analysis remains an invaluable compass; indeed, it guides the way, furthermore enabling precise and purposeful manipulation of matter. Additionally, it offers clarity; moreover, it supports innovation, thus advancing goals. Meanwhile, researchers benefit, similarly applying insights. Consequently, outcomes improve, likewise enhancing accuracy. Hence, it is essential, besides adaptable, ultimately shaping the future.